Abstract
Myeloproliferative neoplasms (MPN) are characterized by dysregulated JAK2 signaling. Clinical type I JAK2 inhibitors as ruxolitinib are hampered by limited disease-modifying effects and occurrence of resistance. Based on pre-clinical data, novel type II JAK2 inhibitors as CHZ868 effectively stabilize inactive JAK2, reduce MPN clones and overcome resistance to ruxolitinib. We study whether MPN cells could escape from type ll JAK2 inhibition, and characterize therapeutic approaches.
To assess potential escape from type II JAK inhibition, JAK2V617F MPN cells incl. SET2, UKE-1 and Ba/F3 EPOR Jak2V617F were exposed long-term to CHZ868. Outgrowing cells showed significantly increased IC50 to CHZ868 and reduced susceptibility for apoptosis induction. No second-site mutations in JAK2 were detected. Phosphoproteomic analysis identified >2500 differentially phosphorylated sites with most prominent activation of the MAPK pathway in JAK inhibitor resistant cells. Type II JAK inhibition persistently suppressed JAK2 - STAT3/5 signaling, while MAPK target activation incl. MEK and ERK was prominent also by phospho-immunoblot suggesting MAPK pathway signaling as a driver of escape from type II JAK inhibition.
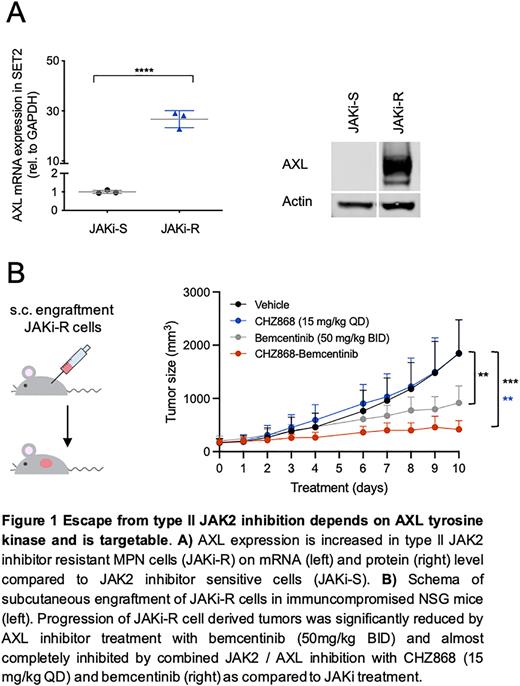
To characterize the molecular basis, we profiled differential gene expression and chromatin accessibility by RNA-sequencing and ATAC-sequencing in MPN cells escaping JAK inhibition. Paired analysis of RNA-seq/ATAC-seq revealed enrichment of H3K27me3 and PRC2 signatures with overall more compacted chromatin. Expression of AXL tyrosine kinase known to signal through the MAPK pathway, was significantly increased along with enriched RAS signaling target gene patterns by GSEA. Motif analysis suggested differential transcriptional programs with loss of ETS and increased accessibility of GATA transcription factor family binding sites. Enhanced AXL expression was confirmed by immunoblotting and depletion of AXL by AXL-specific shRNA knock-down resensitized resistant cells to CHZ868 with decreased IC50. Since these findings pointed towards a role of AXL activation for escape of MPN cells from type II JAK inhibition, we targeted AXL activation using respective inhibitors. AXL inhibitor bemcentinib and AXL/FLT3 inhibitor gilteritinib reduced proliferation of resistant cells with up to 5.6-fold and 3.4-fold decreased IC50, respectively. Given that type II JAK inhibition with CHZ868 maintained suppression of JAK2-STAT3/5 activation in resistant cells, we combined JAK2 and AXL inhibition. Notably, dual JAK2 / AXL inhibition with CHZ868 / bemcentinib or CHZ868 / gilteritinib showed most pronounced inhibitory effects with IC50 similar to values seen in JAK inhibitor sensitive cells and effective induction of apoptosis. Resistant MPN cells engrafted subcutaneously into immunocompromised NSG mice and resulted in robust tumor growth despite JAK2 inhibitor treatment. However, AXL inhibition with bemcentinib reduced progression of type II JAK inhibitor resistant tumors. Combined JAK2 and AXL inhibition showed most pronounced suppression of subcutaneous tumor growth with minimal progression.
Given that inhibitors of MAPK signaling targeting e.g. MEK-ERK activation, are in clinical use for other malignancies with favorable tolerability profiles, we then characterized combined JAK2 / MAPK inhibition to overcome AXL-MAPK driven escape of MPN cells from type II JAK inhibitors. Combination of CHZ868 and MEK inhibitor trametinib significantly reduced subcutaneous tumor growth in NSG mice similarly to JAK2 / AXL inhibition. Expression of MAPK pathway effectors as DUSP6 was substantially reduced. Furthermore, intravenous engraftment of JAK inhibitor resistant cells into NSG mice led to substantial bone marrow (BM) infiltration shown by luciferase imaging and hCD45 immunohistochemistry despite treatment with CHZ868. Combined JAK2 / MAPK inhibition with CHZ868 and trametinib significantly reduced BM infiltration by luciferase imaging and histology supporting that escape from type II JAK inhibition is dependent on AXL-MAPK activation.
In summary, we report on a novel mechanism of AXL-MAPK driven escape from type II JAK2 inhibition which is targetable in vitro and in vivo. Our findings highlight that rationally designed combination therapy approaches can restore sensitivity to JAK inhibitor therapy in MPN, which may have potential implications for other malignant settings.
Disclosures
Angelillo-Scherrer:SNSF: Research Funding; Vaderis: Consultancy; Pfizer: Other: Speaker fees; Silence Therapeutics: Research Funding. Levine:Imago, Mission Bio, Bakx, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics and Isoplexis: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca and Kura: Other: honoraria for invited lectures ; Ajax, Abbvie, Constellation, Zenalis, Celgene, Roche, and Prelude: Other: research support; Syndax, Incyte, Janssen, Astellas, Morphosys and Novartis: Consultancy; Qiagen: Other: supervisory board member; Gilead and Novartis: Other: Grant reviews. Meyer:Novartis and Celgene / BMS: Consultancy; Celgene / BMS: Other: honorarium for invited lecture / webinar.
Author notes
Asterisk with author names denotes non-ASH members.